| The Elements Lithium (Li), Sodium (Na), and Potassium (K) all formed oxides in the ratio of two atoms per oxygen atom: R2O | |
| The Elements Beryllium (Be), Magnesium (Mg), and Calcium (Ca) all formed oxides in the ratio of one atom per oxygen atom: RO | |
| Boron (B) and Aluminum (Al) formed R2O3 | |
| Carbon (C) and Silicon (Si) formed RO2 |
Recognizing the patterns of combining ratios or "valency", Mendeleev created a table organized by placing elements with similar combining ratios in the same group. He arranged the elements within a group in order of their atomic mass.
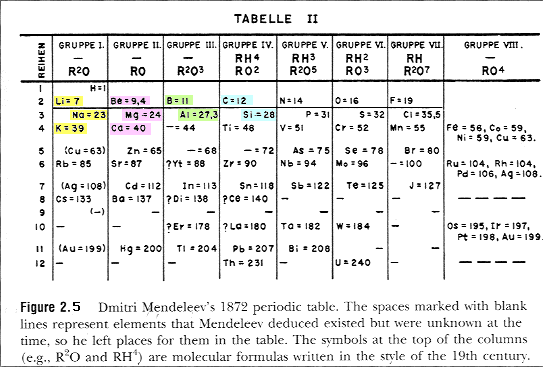 |
In 1869, the Russian chemist Mendeleev noted that the repeating patterns of behavior could be arranged in a sequence of elements giving rise to the "Periodic Table" of the elements. |
Special thanks to Dr. Paul Karol's "Intro to Modern Chemistry" for providing much of the information on this page.
![]()
L O S
A L A M O S N A T I O N A L L A B O R A T O R Y
Operated by the University
of California for the US Department
of Energy
LANL
External View |
www@lanl.gov | Help
| Copyright © UC 1999 | Disclaimer